A cough suppressant causing anaphylaxis? It sounds improbable. But the case reported highlights the need to consider even seemingly benign over-the-counter medications as potential triggers for severe allergic reactions. It's a reminder that in medicine, we must always be prepared for the unexpected, even with medications we consider safe.
This isn't about fear-mongering; it's about sharpening our diagnostic acumen. How do we distinguish a true drug allergy from a simple side effect? And when do we pursue confirmatory testing, like skin tests, to guide future avoidance?
Clinical Key Takeaways
lightbulb
- The PivotEven common over-the-counter medications like dextromethorphan can cause severe allergic reactions.
- The DataPositive skin testing can confirm suspected dextromethorphan allergy, guiding future avoidance strategies.
- The ActionIn patients presenting with anaphylaxis of unclear etiology, consider dextromethorphan as a potential trigger and pursue allergy testing.
Presentation
A patient presents with acute anaphylaxis following ingestion of an over-the-counter cough syrup. We've all seen it - the rapid onset of urticaria, angioedema, respiratory distress, and hypotension. The immediate response is always the same: epinephrine, antihistamines, and corticosteroids. But after the acute crisis is managed, the real detective work begins: what triggered this?
The usual suspects are considered: food, insect stings, medications. But what if the trigger is an unexpected component of that seemingly harmless cough syrup? This is the conundrum presented by the case report: anaphylaxis to dextromethorphan, a common ingredient in many cough remedies.
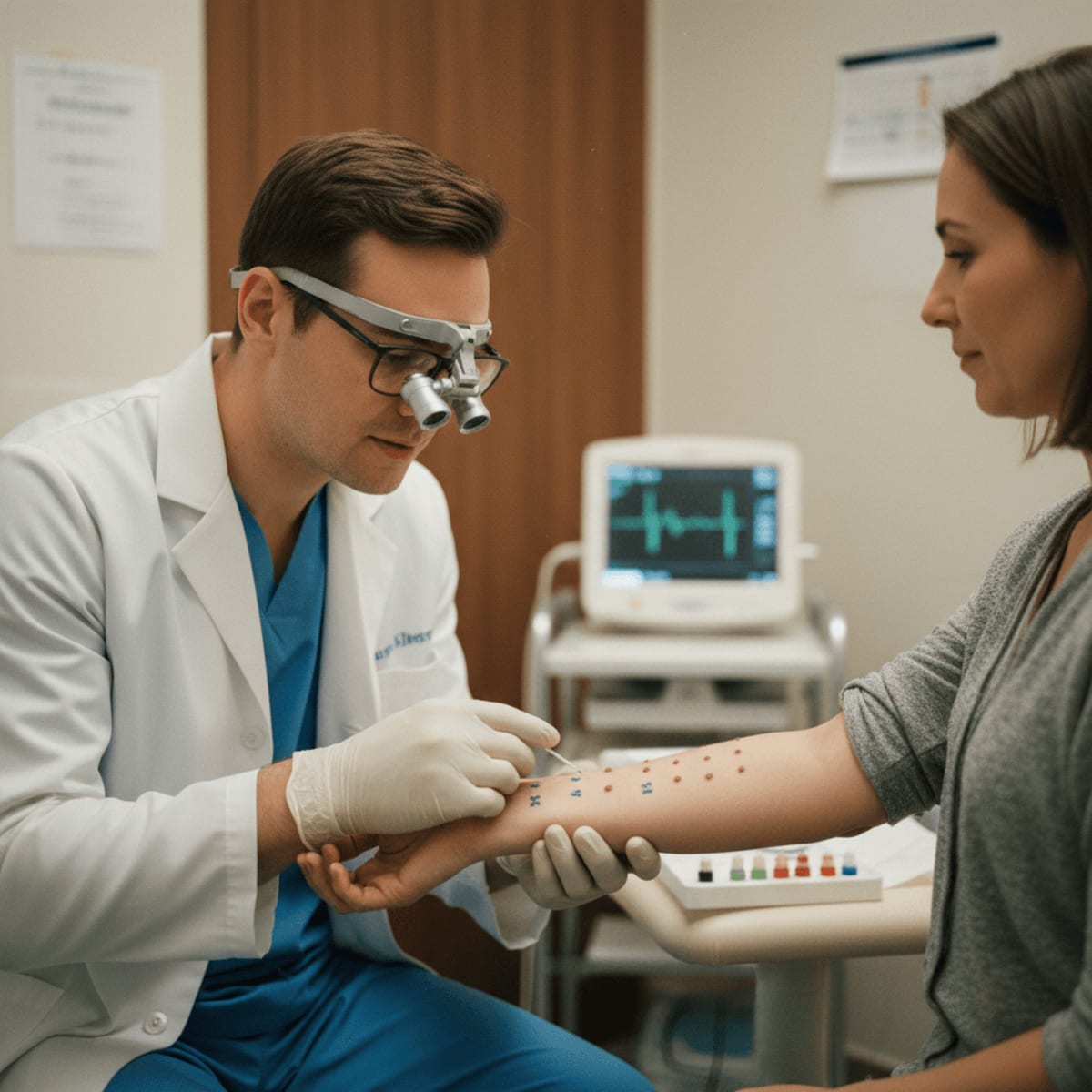
Diagnostic Workup
The diagnostic process involves a thorough history, but recall bias and incomplete ingredient lists often muddy the waters. This case emphasizes the value of directed allergy testing. Skin prick tests, followed by intradermal testing, can help pinpoint the specific allergen responsible. A positive skin test to dextromethorphan, as demonstrated in this case, provides strong evidence of a true drug allergy. It allows us to inform the patient to avoid all products containing dextromethorphan.
Now, are we to skin test everyone who reports a possible reaction to a cough syrup? Of course not. Clinical judgment is paramount. But in cases of unexplained anaphylaxis, particularly when a temporal relationship to medication ingestion is suspected, allergy testing becomes a valuable tool. This approach aligns with the general principles outlined in the NIAID guidelines for the diagnosis and management of food allergy, which emphasize component-resolved diagnostics to identify specific allergenic proteins. We adapt those methods to drug hypersensitivity.
A critical point to consider: the negative predictive value of skin testing. A negative skin test does NOT definitively rule out drug allergy. Non-IgE mediated mechanisms can also be at play, requiring different diagnostic approaches, such as drug provocation testing under controlled settings.
The Catch: Study Limitations
Let's be clear: a single case report is hypothesis-generating, not practice-changing. We cannot extrapolate broad conclusions based on one patient's experience. Furthermore, the absence of a standardized protocol for dextromethorphan skin testing introduces variability in the sensitivity and specificity of the test. Did they use the correct concentration? Was the technique flawless? The report lacks detail about these crucial aspects. This is not a randomized controlled trial; it's a clinical observation. Its value lies in raising awareness and prompting further investigation.
Also, who funded this report? Was it supported by a pharmaceutical company with a vested interest in promoting alternative cough remedies? Transparency is essential. The lack of information regarding funding sources casts a shadow of doubt on the impartiality of the findings.
Clinical Implications
The cost of allergy testing can be substantial, and insurance coverage may vary. A single drug allergy workup can easily run into thousands of dollars, placing a significant financial burden on patients. Furthermore, the time required for testing and interpretation can disrupt clinic workflow and delay diagnosis.
From a risk management perspective, documenting a confirmed dextromethorphan allergy in a patient's chart is crucial. This information should be prominently displayed to prevent accidental administration of the drug in the future. This requires careful attention to detail and effective communication between healthcare providers.
Finally, consider the medicolegal ramifications. Failure to recognize and appropriately manage a drug allergy can lead to adverse outcomes and potential litigation. Clinicians must stay informed about potential drug allergies and implement strategies to minimize the risk of allergic reactions.
The financial toxicity of diagnostic testing and the workflow impact on busy clinics cannot be ignored. Clear protocols and accessible testing are needed to avoid disparities in care.
Having a system for flagging confirmed drug allergies in electronic health records is critical to prevent future adverse events.
LSF-6745417394 | December 2025

How to cite this article
Webb M. Dextromethorphan anaphylaxis the diagnostic puzzle. The Life Science Feed. Published January 5, 2026. Updated January 5, 2026. Accessed January 31, 2026. .
Copyright and license
© 2026 The Life Science Feed. All rights reserved. Unless otherwise indicated, all content is the property of The Life Science Feed and may not be reproduced, distributed, or transmitted in any form or by any means without prior written permission.
Fact-Checking & AI Transparency
This summary was generated using advanced AI technology and reviewed by our editorial team for accuracy and clinical relevance.
References
- NIAID. (2010). Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. Journal of Allergy and Clinical Immunology, 126(6 Suppl), S1-S58.
- Kelso, J. M. (2014). Drug allergy: diagnosis and management. Journal of Allergy and Clinical Immunology, 133(2), 321-329.
- Joint Task Force on Practice Parameters. (2017). Drug allergy: an updated practice parameter. Annals of Allergy, Asthma & Immunology, 119(6), 620-628.